
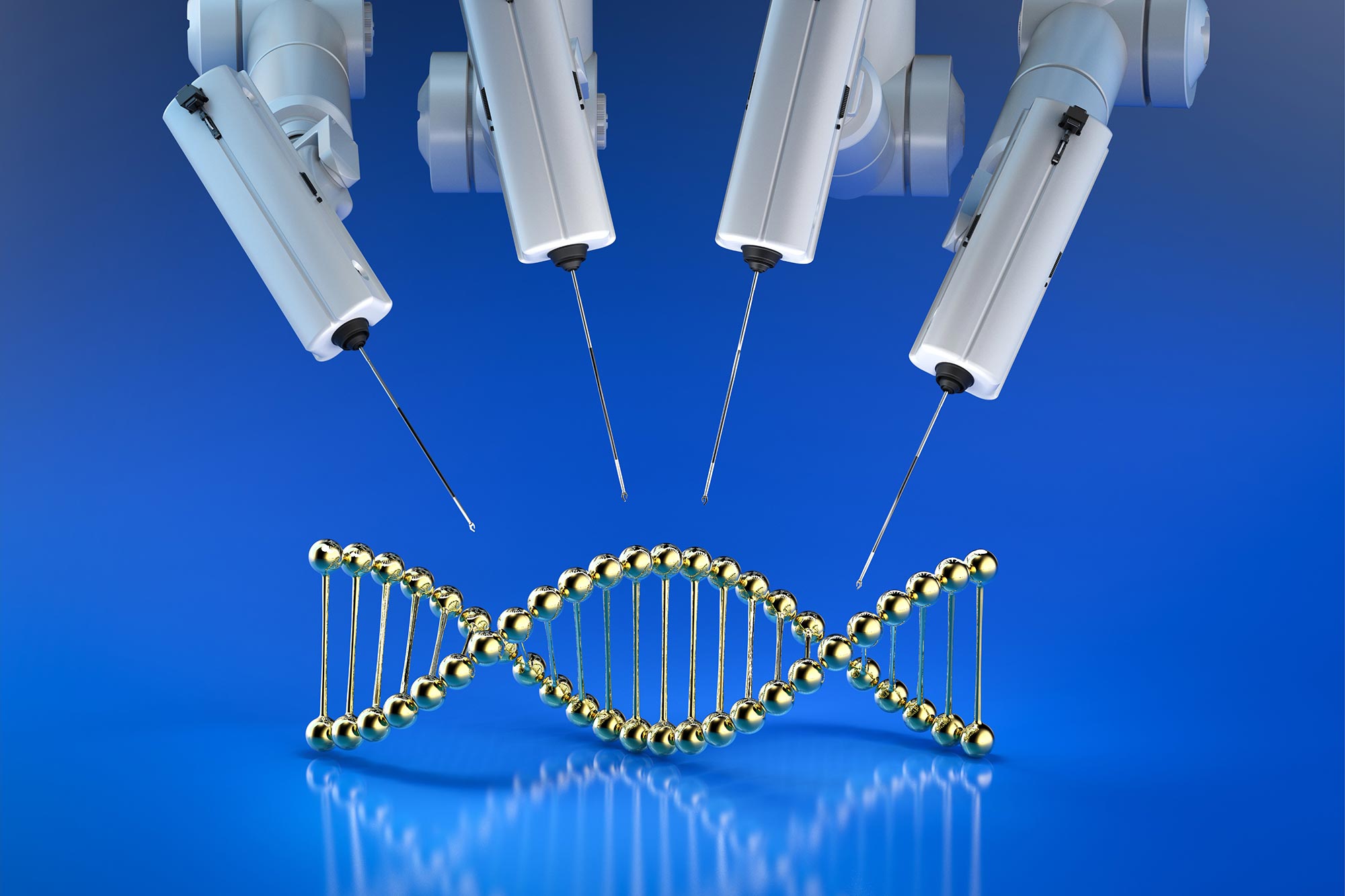
カリフォルニア大学サンタバーバラ校の研究者らは、遺伝子テンプレートの送達にウイルス物質を使用せずに、CRISPR/Cas9遺伝子編集の効率を劇的に向上させる方法を開発した。 に掲載された論文で説明されている方法 ネイチャーバイオテクノロジーは、架橋を使用して遺伝子編集プロセスのステップである相同性指向修復を触媒し、変異頻度を増加させることなく効率を 3 倍に高めます。 がんの化学療法で一般的に使用されるこれらの架橋は、細胞の自然な修復機構を強化し、遺伝子編集の成功の可能性を高めることがわかっています。
研究者らは、送達にウイルス物質を使用せずに、クロスリンクを使用して CRISPR/Cas9 遺伝子編集の効率を 3 倍に高めました。 このアプローチは細胞の自然な修復機構を強化し、より正確かつ効率的な遺伝子編集を可能にし、疾患研究と前臨床研究を改善する可能性があります。
遺伝子編集は研究と治療の両方に効果的な方法です。 2012 年に発見された、ノーベル賞を受賞した高速かつ正確なゲノム編集ツールである CRISPR/Cas9 テクノロジーの出現以来、科学者たちはその機能を探索し、そのパフォーマンスを向上させることに取り組んできました。
カリフォルニア大学サンタバーバラ校の生物学者クリス・リチャードソン研究室の研究者らは、この成長を続けるツールボックスに、標的遺伝子配列の編集に使用される遺伝子テンプレートを提供するためにウイルス材料を使用せずにCRISPR/Cas9編集の効率を高める方法を追加した。 雑誌に掲載された彼らの新しい論文によると、 ネイチャーバイオテクノロジー彼らの方法は、「突然変異頻度を増加させたり、末端ドッキング修復の結果を変えたりすることなく」、相同性指向修復(遺伝子編集プロセスの一段階)を約3倍誘導する。
「私たちは非ウイルス性遺伝子編集を改善する化学修飾を発見し、興味深い新しいタイプの遺伝子編集も発見しました。[{” attribute=””>DNA repair,” Richardson said.
Find, Cut and Paste
The CRISPR/Cas9 method works by capitalizing on a defense technique employed by bacteria against viral attackers. To do this, the bacteria snip a piece of the invading virus’s genetic material, and incorporate it into their own in order to recognize it later. Should the bacteria get reinfected, they can target the now-familiar genetic sequences for destruction.
In gene editing, this process uses the enzyme Cas9 as molecular “scissors” to snip sequences it recognizes, guided by the CRISPR system. This cut is also an opportunity to replace the severed genes with similar (homologous) but improved ones, utilizing the cell’s natural repair mechanisms. If successful, the cell should have modified expressions and functions thereafter.
To deliver the repair template DNA to the nucleus of the cell where its genetic material lives, oftentimes viruses are used. While they are effective, the researchers say, viral workflows “are expensive, difficult to scale and potentially toxic to cells.”
Nonviral templates are potentially less expensive and more scalable, although researchers still must overcome efficiency and toxicity barriers. In their study, the Richardson Lab found that introducing interstrand crosslinks into the workflow increased homology directed repair dramatically.
“Every workflow that we have put this approach into has worked better by roughly threefold,” Richardson said.
Interstrand crosslinks are lesions that keep the double strands of a DNA helix tethered to each other, making them unable to replicate. Cancer chemotherapies use this mechanism to interrupt tumor growth and kill cancer cells. Added to a homology directed repair template, however, these crosslinks were found to stimulate the cell’s natural repair mechanisms and increase the likelihood of editing success.
“Basically, what we’ve done is taken this template DNA and damaged it,” Richardson said. “We’ve in fact damaged it in the most severe way I can think of. And the cell doesn’t say, ‘Hey this is junk; let me throw it away.’ What the cell actually says is, ‘Hey this looks great; let me stick it into my genome.’” The result is a highly efficient and minimally error-prone nonviral system of gene editing.
Their discovery, like many breakthroughs in science, was actually something of a happy accident. While working to purify proteins to study DNA repair, graduate student researcher and lead author Hannah Ghasemi noted unanticipated changes to the outcomes of their experiments.
“We were introducing these chemical modifications to the DNA templates in order to be able to pull them out of the cells and see what proteins were bound to them, and I was just checking to see if this modification had somehow affected the editing in any capacity,” she said. “I was expecting to either see no change or that it actually might have negatively affected the editing.”
What she found instead was a positive effect, up to three times the editing activity of the uncrosslinked controls. Furthermore, the team found that even with the increase in edits — and therefore the chances for errors — there was no increase in mutation frequency. They are still investigating the specific mechanisms leading to this result, but they have ideas.
“What we think happens is that the cell detects and tries to repair the damaged DNA that we’ve added this crosslink to,” Richardson said. “And in doing so, it delays the cell past a checkpoint where it would normally stop this recombination process. And so by prolonging the amount of time that it takes the cell to do this recombination, it makes it more likely that the edits will go to completion.” Studying this new process could also lead to a better understanding about how cells detect editing reagents and how they “decide” to accept them or not, he said.
This method will find the most use in ex-vivo gene editing applications, according to the team, that is, in the realm of disease research and preclinical work.
“We can more effectively knock down genes and insert things into genomes to study systems outside of the human body in a lab setting,” Ghasemi said. This development allows them to more efficiently build disease models and test hypotheses about how diseases work, which could lead to better clinical and therapeutic approaches.
Reference: “Interstrand crosslinking of homologous repair template DNA enhances gene editing in human cells” by Hannah I. Ghasemi, Julien Bacal, Amanda C. Yoon, Katherine U. Tavasoli, Carmen Cruz, Jonathan T. Vu, Brooke M. Gardner and Chris D. Richardson, 27 February 2023, Nature Biotechnology.
DOI: 10.1038/s41587-022-01654-y

「アマチュア主催者。ビールの伝道者になりたい。一般的なウェブファン。認定インターネット忍者。熱心な読者。」





More Stories
スペースXのファルコン9ロケットが打ち上げ前に停止、億万長者が特別任務に就く
ブラックホールはどのようにしてこれほど大きく、そして速く成長したのでしょうか?答えは暗闇の中にあります
世界最速の顕微鏡が電子の動きをアト秒で捉える:ScienceAlert